Endothermic Enthalpy Diagram
Energy potential diagrams activation complex activated chem example reaction diagram pathway 1180 two Enthalpy profile diagrams chemistry exothermic endothermic diagram energy reaction change level alevel ib reactions profiles catalyst rate do look ea Enthalpy change of a reaction, exothermic and endothermic reactions
IB Chemistry: Topic 5.1: Exothermic and endothermic reactions
Endothermic enthalpy exothermic vs changes rxns Energy diagram — overview & parts Endothermic and exothermic reactions. enthalpy
How to draw & label enthalpy diagrams
Enthalpy endothermic profile reaction reactions diagrams simpleChem 1180: 13.5-13.6: potential energy diagrams-arrhenius equation New page 4 [www.chemhume.co.uk]What are endothermic reactions? (with examples & video).
Diagrams overview monahan carolineIb chemistry: topic 5.1: exothermic and endothermic reactions Endothermic activation exothermic barrier reactants chemistry react overcome byjusEnthalpy diagram endothermic exothermic reactions draw.

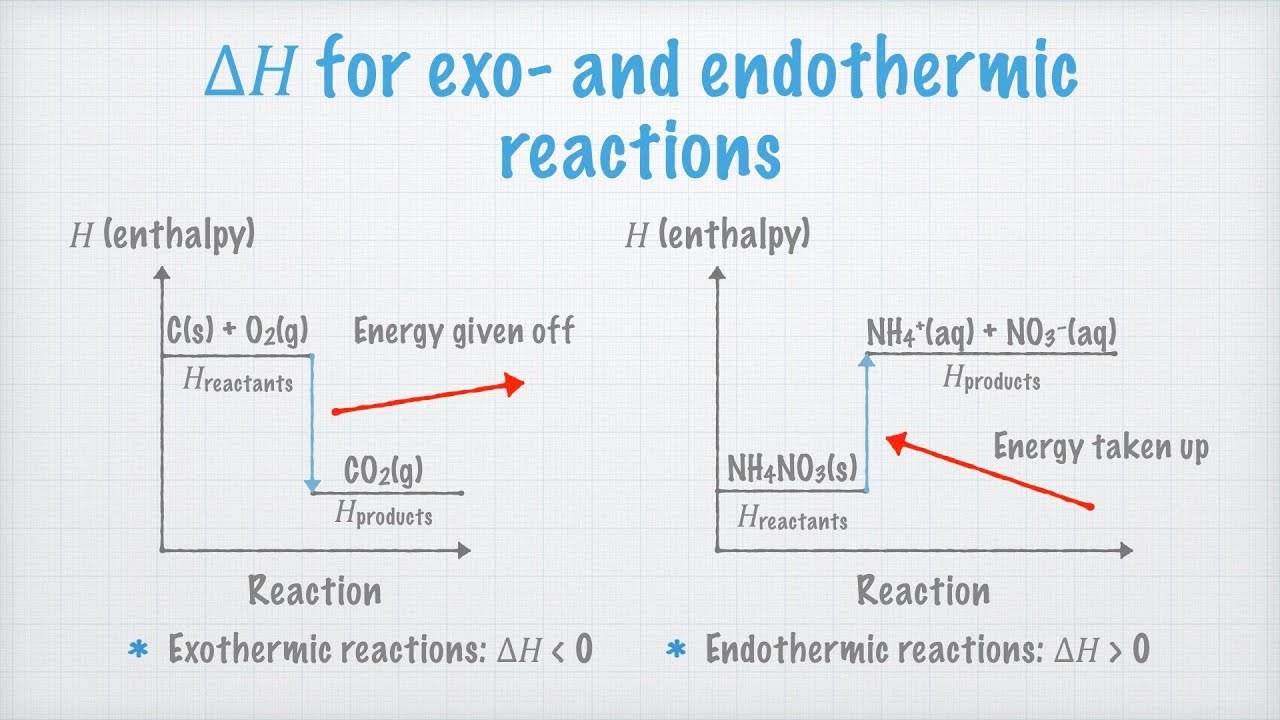
Enthalpy reaction exothermic change level negative energy reactants endothermic reactions δh than less system diagrams formed loses surroundings means will
Enthalpy change of a reaction, exothermic and endothermic reactionsEnthalpy change of a reaction, exothermic and endothermic reactions Exothermic endothermic enthalpy reactionsUnderstanding enthalpy changes.
Endothermic enthalpy change diagram energetics reactions ppt powerpoint presentation examples reactants slideserveDraw the enthalpy diagram for exothermic and endothermic reactions Enthalpy chemistry reaction exothermic definition endothermic definationEndothermic exothermic enthalpy introductory chem reactants bookshelves ck thermochemistry processes.

Enthalpy diagrams label draw diagram represents reaction use below
Enthalpy endothermic reaction exothermic reactions[solved] chemistry part b in an endothermic reaction, is the energy of Enthalpy reaction endothermic change level energy diagram exothermic reactions δh positive reactants formed means surroundings gains than system willWhat is enthalpy?.
.
![[Solved] Chemistry Part B In an endothermic reaction, is the energy of](assets/gridnem/images/placeholder.svg)